Abstract

Background: Von Willebrand Disease (VWD) is the most common inherited bleeding disorder, which results from either a quantitative (Type 1 or 3) or qualitative (Type 2) defect in von Willebrand Factor (VWF). Prior studies, largely focused on individuals with mild VWD and bleeding symptoms, have shown significant reductions in quality of life in patients with Von Willebrand disease. Because of the rarity less is known about individuals with severe forms of VWD or those with a severe bleeding phenotype.
Aim: To report quality of life measures in participants with clinically severe VWD in the United States (US).
Study Design and Methods: ATHN 9 Severe VWD Natural History Study is sponsored by the American Thrombosis and Hemostasis Network (ATHN) and is being conducted at 25 ATHN-affiliated sites across the US. ATHN 9 is an observational study focused on persons with clinically severe VWD treated with VWF factor on demand or for prophylaxis. Participants are identified by site investigators with a projected goal to enroll 130 individuals. Patients with severe VWD defined as type 3 VWD, or a VWF:RCo, VWF:GPIbM or VWF:Ag ≤ 30%, or persons with "clinically severe VWD" defined by VWF:RCo, VWF:GPIbM or VWF:Ag ≤ 40% and requiring recurrent use of factor concentrates, and prior enrollment in the ATHNdataset national surveillance data collection project are included. Participants with platelet-type or acquired VWD are excluded. Quality of life was assessed using PROMIS measures (PROMIS 25, PROMIS 25 Proxy, PROMIS 29) and the VWIQ at enrollment, annually, and at study exit. PROMIS-29 assesses seven different quality of life domains (physical function, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles, and pain interference) and has been validated in adult populations. PROMIS measures in the general US population have a mean of 50 and standard deviation of 10. PROMIS-25 is a pediatric questionnaire assessing the same domains (except for sleep disturbance) and an accompanying proxy form for caregivers of participants <8 years of age. Baseline enrollment results are reported here.
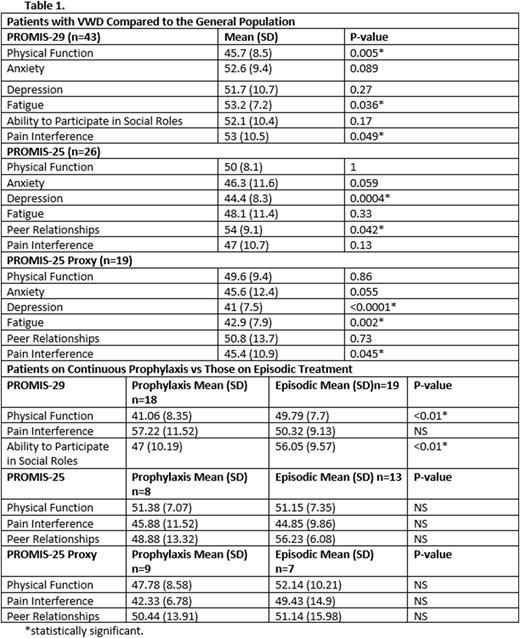
Results: Enrollment (baseline) PROMIS-29 forms were completed by 43 participants. At baseline, mean physical function was lower than the general population at 45.7 (p=0.005), and fatigue and pain interference were greater than the general population at 53.2 and 53 respectively (p=0.036 and 0.049 respectively). Individuals on continuous VWF factor prophylaxis (n=18) had significantly decreased physical function (p<0.01), and decreased ability to participate in social roles (p<0.01) than individuals on episodic treatment (n=19). Enrollment PROMIS-25 forms were completed for 26 participants, while PROMIS-25 proxy forms were completed for 19 participants. Mean enrollment scores for PROMIS-25 depression symptoms was lower than the general population at 44.4 (p=0.0004) and peer relationships were better than the general population at 54 (p=0.042). Mean enrollment scores for PROMIS-25 Proxy were lower than the general population for depression, fatigue, and pain interference at 41, 42.9, and 45.4 respectively (p<0.0001, p=0.002, and p=0.045 respectively). In contrast to the adult participants, no statistically significant differences were seen between pediatric participants on VWF factor prophylaxis versus episodic treatment.
Discussion: Pediatric participants with clinically severe VWD reported less depression and better peer relationships than the general population, and parents of pediatric participants with VWD reported less depression, fatigue, and pain interference in their children compared to the general population. Patient-reported outcomes in adult participants were less encouraging with worse physical function, and more fatigue and pain interference compared to the general population. Adult participants on VWF factor prophylaxis had decreased physical function, and decreased ability to participate in social roles compared to adults treated episodically, perhaps secondary to disease severity and accumulation of bleed-related morbidity. No statistically significant differences were seen in quality of life outcomes between pediatric patients on VWF factor prophylaxis and those on episodic treatment.
Disclosures
Weyand:Pfizer: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Spark: Consultancy; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Recht:Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Oregon Health & Science University: Ended employment in the past 24 months; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment. Sidonio, Jr.:Guardian Therapeutics: Honoraria; Novo Nordisk: Honoraria; Bayer: Honoraria; UniQure: Honoraria; Biomarin: Honoraria; Octapharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Spark: Honoraria; Genentech: Honoraria, Research Funding; Pfizer: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal